Abstract
Introduction: Adoptive cell therapy with chimeric antigen receptor (CAR) T-cells has improved responses in refractory diffuse large B-cell lymphoma (DLBCL) and multiple myeloma (MM). Most CAR-T recipients experience cytokine release syndrome (CRS) and immune effector cell-associated neurotoxicity syndrome (ICANS) - similar yet clinically distinct inflammatory syndromes related to T-cell activation and expansion of multiple cytokines. Differences in tumor biology between MM and DLBCL and how they contribute to differences in cytokine expression is not well understood. Given different CAR T-cell constructs have varying levels of activation and toxicity, cytokine profiles between products and between different disease entities may inform management. In this study, we used multiplex proteomic profiling to evaluate differences in plasma cytokine signatures between two CAR T-cell products and diseases.
Methods: Between September 2021 and March 2022, 20 patients received CAR T-cell therapy and consented to the Yale School of Medicine hematologic disease tissue bank (HIC#1401013259). Two longitudinal cohorts were prospectively enrolled: (1) patients who received lisocabtagene maraleucel (liso-cel) for DLBCL refractory after two lines of therapy, and (2) those who received idecabtagene vicleucel (ide-cel) for MM refractory after 4 lines of therapy. For each patient, the first sample was collected prior to lymphodepleting chemotherapy on day -5 with subsequent samples collected on days 0 (prior to CAR T-cell infusion) and 1, 2, 3, and 7 (after CAR T-cell infusion). Plasma samples were processed and frozen within 1 hour of collection. Proteomic profiling was performed at Eve Technologies (Calgary, Alberta, Canada). The Human 71-Plex Discovery Assay measuring 71 total cytokines and chemokines was used to interrogate each sample time point. Statistical analysis was performed in GraphPad Prism (GraphPad Software, San Diego, CA) and R (R Core Team). Levels of individual proteins were compared using Wilcoxon tests. P-values <0.05 were considered statistically significant.
Results: Among 13 patients who received liso-cel for DLBCL (including 2 with transformed follicular lymphoma and 3 with double/triple hit pathology), the median age was 66 years (44-82), 7 (54%) were males, 8 (62%) had KPS ≥ 90, 8 (62%) had bulky disease, median lines of therapy were 4 (2-10), and 11 (85%) received systemic bridging therapy. Seven patients (54%) had any grade CRS, with one patient having grade 3 CRS; 2 (15%) had grade 1 and 2 ICANS. Four patients (31%) received an IL-6 inhibitor. At 3 months of follow-up, the objective response rate was 77% (10/13), of whom two patients had a complete response and 60% (6/10) had any grade CRS.
Among 7 patients who received ide-cel for MM, with R-ISS score of 1, 2, and 3 in 4 (57%), 1 (14%), and 2 (29%) patients, respectively, the median age was 61 years (54-79), 5 (71%) were males, 4 (57%) had KPS ≥ 90, 3 (43%) had extramedullary disease, median lines of therapy were 7 (4-13), and 5 patients (71%) received systemic bridging therapy. Six patients (86%) had any grade CRS with no patients having grade ≥ 3 CRS; 3 (43%) had grade 1 ICANS. Four patients (57%) received an IL-6 inhibitor. Five patients (63%) received growth factor support on day 7. At 3 months of follow-up, the objective response rate was 86% (6/7) of whom two patients had a complete response and 83% (5/6) had any grade CRS.
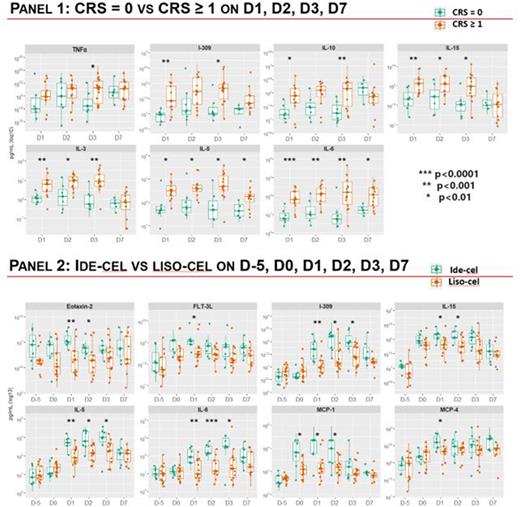
In a longitudinal analysis, significantly higher levels of IL-3, IL-5, IL-6, IL-10, IL-15, IL-309, and TNFα were noted during CRS (panel 1). In a comparison between CAR constructs, ide-cel was characterized by significantly higher levels of Eotaxin-2, FLT-3L, IL-5, IL-6, IL-15, I-309, MCP-1, and MCP-4 compared to liso-cel (panel 2). Many of these elevated cytokine proteins are implicated as chemoattractants for other leukocyte subtypes including but not limited to neutrophils, monocytes, eosinophils which may contribute to inflammation and may modulate response, toxicity, or relapse.
Conclusions: Ide-cel is associated with a distinct cytokine and chemokine signature compared to liso-cel. This study provides pre-clinical evidence for the role of IL-5 inhibitors to treat or prevent CRS following MM-directed CAR T-cell therapy. Further studies with larger cohorts of patients are needed to validate these findings.
Disclosures
Neparidze:GSK: Research Funding; Janssen: Research Funding. Foss:Kyowa: Consultancy; Daiichi: Consultancy; Conjupro: Consultancy; Astex: Consultancy; Seagen: Consultancy, Speakers Bureau. Isufi:Bayer: Honoraria; ADC Therapeutics: Membership on an entity's Board of Directors or advisory committees; BEAM Therapeutics: Membership on an entity's Board of Directors or advisory committees; Kite: Speakers Bureau; Epizyme: Membership on an entity's Board of Directors or advisory committees. Halene:Forma Therapeutics: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal